- Lewis Dot Diagram Solver
- Lewis Dot Structure Calc
- Lewis Electron Dot Structure Calculator Equation
- Lewis Electron Dot Structure Calculator Function
Rules for Drawing Lewis Dot Structure for Molecules 1) Calculate (add up) the total number of Valence Electrons from all atoms a. It does not matter which atom the electrons come from b. Add 1 e for each (-) charge (anions) c. Subtract 1 e for each (+) charge (cation) a. 2) Identify the Central and Terminal Atoms 1 H and F → always terminal b.
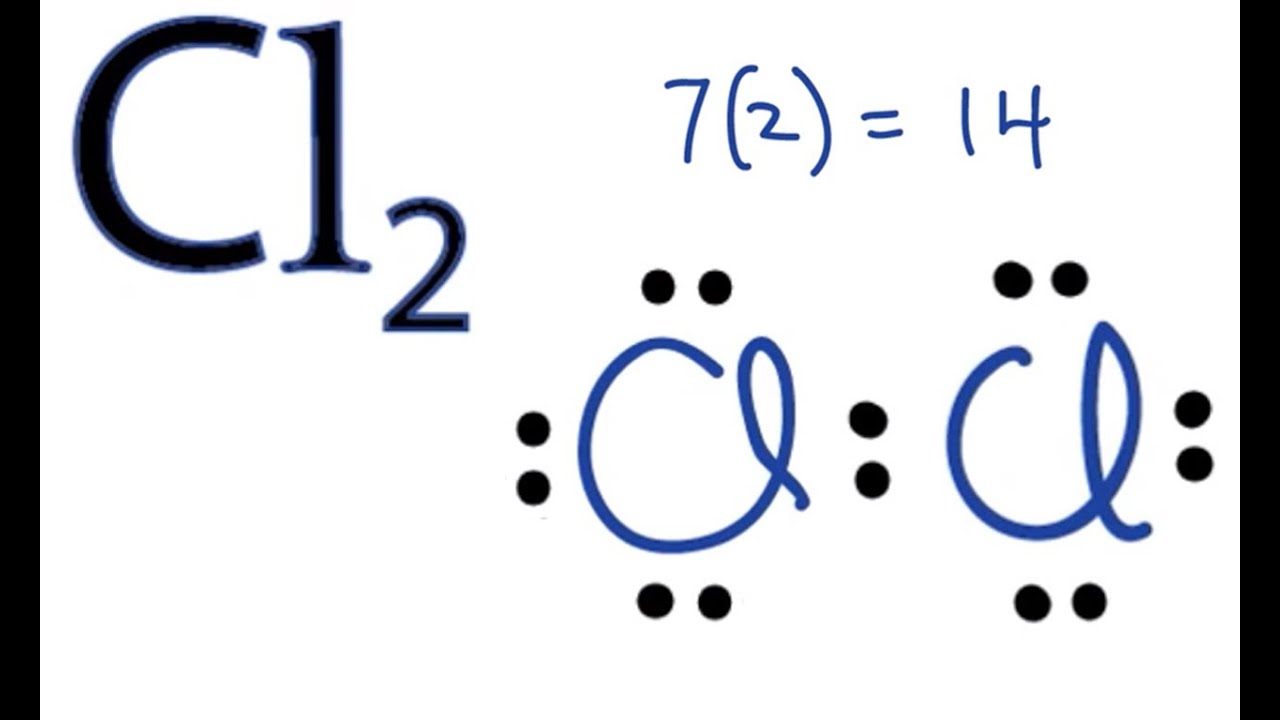
- Lewis dot structures help predict molecular geometry. How Can I Spread The Dots Around Iodine Atome How To Draw The Dot Structure For I2 The Basics Of Chemical Bonding How To Draw The Lewis Dot Structure For I- (Iodide Ion. I2 lewis structure how to draw the dot structure for i2 a step by step explanation of how to write the lewis dot structure for i2 iodine gas for the i2 lewis.
- The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons.
- Equivalent Lewis dot structures, such as those of ozone, are called resonance structures A Lewis electron structure that has different arrangements of electrons around atoms whose positions do not change. The position of the atoms is the same in the various resonance structures of a compound, but the position of the electrons is different.
Using Lewis Dot Symbols to Describe Covalent Bonding
This sharing of electrons allowing atoms to “stick” together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0.1 nm, or if you prefer 100 pm, at which the attractive forces significantly outweigh the repulsive forces and a bond will be formed if both atoms can achieve a completen s2np6 configuration. It is this behavior that Lewis captured in his octet rule. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl2, they can each complete their valence shell:
Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pair ; the other three pairs of electrons on each chlorine atom are called lone pairs. Lone pairs are not involved in covalent bonding. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond.
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols:
The structure on the right is the Lewis electron structure, or Lewis structure, for H2O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Chemists usually indicate a bonding pair by a single line, as shown here for our two examples:
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:
- Arrange the atoms to show specific connections. When there is a central atom, it is usually the least electronegative element in the compound. Chemists usually list this central atom first in the chemical formula (as in CCl4 and CO32−, which both have C as the central atom), which is another clue to the compound’s structure. Hydrogen and the halogens are almost always connected to only one other atom, so they are usually terminal rather than central.
Note:
The central atom is usually the least electronegative element in the molecule or ion; hydrogen and the halogens are usually terminal.
- Determine the total number of valence electrons in the molecule or ion. Add together the valence electrons from each atom. (Recall from Chapter 2 that the number of valence electrons is indicated by the position of the element in the periodic table.) If the species is a polyatomic ion, remember to add or subtract the number of electrons necessary to give the total charge on the ion. For CO32−, for example, we add two electrons to the total because of the −2 charge.
- Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. In H2O, for example, there is a bonding pair of electrons between oxygen and each hydrogen.
- Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). These electrons will usually be lone pairs.
- If any electrons are left over, place them on the central atom. Some atoms are able to accommodate more than eight electrons.
- If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple (double or triple) bonds to the central atom to achieve an octet. This will not change the number of electrons on the terminal atoms.
Now let’s apply this procedure to some particular compounds, beginning with one we have already discussed.
H2O
1. Because H atoms are almost always terminal, the arrangement within the molecule must be HOH.
2. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons
3. Placing one bonding pair of electrons between the O atom and each H atom gives H:O:H, with 4 electrons left over.
4. Each H atom has a full valence shell of 2 electrons.
5. Adding the remaining 4 electrons to the oxygen (as two lone pairs) gives the following structure:
This is the Lewis structure we drew earlier. Because it gives oxygen an octet and each hydrogen two electrons, we do not need to use step 6.
1. With only two atoms in the molecule, there is no central atom.
2. Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons.
3. Placing a bonding pair of electrons between O and Cl gives O:Cl, with 12 electrons left over.
4. If we place six electrons (as three lone pairs) on each atom, we obtain the following structure:
Each atom now has an octet of electrons, so steps 5 and 6 are not needed. The Lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. OCl− is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant.
1. Because carbon is less electronegative than oxygen and hydrogen is normally terminal, C must be the central atom. One possible arrangement is as follows:
2. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons.
3. Placing a bonding pair of electrons between each pair of bonded atoms gives the following:
Six electrons are used, and 6 are left over.
4. Adding all 6 remaining electrons to oxygen (as three lone pairs) gives the following:
Although oxygen now has an octet and each hydrogen has 2 electrons, carbon has only 6 electrons.
5. There are no electrons left to place on the central atom.
6. To give carbon an octet of electrons, we use one of the lone pairs of electrons on oxygen to form a carbon–oxygen double bond:
Both the oxygen and the carbon now have an octet of electrons, so this is an acceptable Lewis electron structure. The O has two bonding pairs and two lone pairs, and C has four bonding pairs. This is the structure of formaldehyde, which is used in embalming fluid.
An alternative structure can be drawn with one H bonded to O. Formal charges, discussed later in this section, suggest that such a structure is less stable than that shown previously.
Example
Write the Lewis electron structure for each species.
- NCl3
- S22−
- NOCl
Given: chemical species
Asked for: Lewis electron structures
Strategy:
Use the six-step procedure to write the Lewis electron structure for each species.
Show AnswerNitrogen trichloride is an unstable oily liquid once used to bleach flour; this use is now prohibited in the United States.
In a diatomic molecule or ion, we do not need to worry about a central atom. Each sulfur atom (group 16) contains 6 valence electrons, and we need to add 2 electrons for the −2 charge, giving a total of 14 valence electrons. Using 2 electrons for the S–S bond, we arrange the remaining 12 electrons as three lone pairs on each sulfur, giving each S atom an octet of electrons:
Because nitrogen is less electronegative than oxygen or chlorine, it is the central atom. The N atom (group 15) has 5 valence electrons, the O atom (group 16) has 6 valence electrons, and the Cl atom (group 17) has 7 valence electrons, giving a total of 18 valence electrons. Placing one bonding pair of electrons between each pair of bonded atoms uses 4 electrons and gives the following:
Adding three lone pairs each to oxygen and to chlorine uses 12 more electrons, leaving 2 electrons to place as a lone pair on nitrogen:
Because this Lewis structure has only 6 electrons around the central nitrogen, a lone pair of electrons on a terminal atom must be used to form a bonding pair. We could use a lone pair on either O or Cl. Because we have seen many structures in which O forms a double bond but none with a double bond to Cl, it is reasonable to select a lone pair from O to give the following:
All atoms now have octet configurations. This is the Lewis electron structure of nitrosyl chloride, a highly corrosive, reddish-orange gas.
Example
Write Lewis electron structures for CO2 and SCl2, a vile-smelling, unstable red liquid that is used in the manufacture of rubber.
It is sometimes possible to write more than one Lewis structure for a substance that does not violate the octet rule, as we saw for CH2O, but not every Lewis structure may be equally reasonable. In these situations, we can choose the most stable Lewis structure by considering the formal charge on the atoms, which is the difference between the number of valence electrons in the free atom and the number assigned to it in the Lewis electron structure. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. A formal charge does not represent a true charge on an atom in a covalent bond but is simply used to predict the most likely structure when a compound has more than one valid Lewis structure.

To calculate formal charges, we assign electrons in the molecule to individual atoms according to these rules:
- Nonbonding electrons are assigned to the atom on which they are located.
- Bonding electrons are divided equally between the bonded atoms.
For each atom, we then compute a formal charge:
formal charge = valence e−−(free atom)(non−bonding e− + bonding e−/2)
To illustrate this method, let’s calculate the formal charge on the atoms in ammonia (NH3) whose Lewis electron structure is as follows:
A neutral nitrogen atom has five valence electrons (it is in group 15). From its Lewis electron structure, the nitrogen atom in ammonia has one lone pair and shares three bonding pairs with hydrogen atoms, so nitrogen itself is assigned a total of five electrons [2 nonbonding e− + (6 bonding e−/2)]. Substituting into the below equation, we obtain:
formal charge(N)= 5 valence e–−(2non−bonding e− + 6 bonding e−/2)=0
A neutral hydrogen atom has one valence electron. Each hydrogen atom in the molecule shares one pair of bonding electrons and is therefore assigned one electron [0 nonbonding e− + (2 bonding e−/2)]. Using the below equation to calculate the formal charge on hydrogen, we obtain:
formal charge(H)= 1 valence e–−(0 non−bonding e− + 2 bonding e−/2)=0
The hydrogen atoms in ammonia have the same number of electrons as neutral hydrogen atoms, and so their formal charge is also zero. Adding together the formal charges should give us the overall charge on the molecule or ion. In this example, the nitrogen and each hydrogen has a formal charge of zero. When summed the overall charge is zero, which is consistent with the overall charge on the NH3 molecule.
Typically, the structure with the most charges on the atoms closest to zero is the more stable Lewis structure. In cases where there are positive or negative formal charges on various atoms, stable structures generally have negative formal charges on the more electronegative atoms and positive formal charges on the less electronegative atoms. The next example further demonstrates how to calculate formal charges.
Example
Calculate the formal charges on each atom in the NH4+ ion.
Given: chemical species
Asked for: formal charges
Strategy:
Identify the number of valence electrons in each atom in the NH4+ ion. Use the Lewis electron structure of NH4+ to identify the number of bonding and nonbonding electrons associated with each atom and then use the given formula to calculate the formal charge on each atom.
Show AnswerThe Lewis electron structure for the NH4+ ion is as follows:
The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using the formula, the formal charge on the nitrogen atom is
formal charge(N)=5−(0+8/2)=1
Each hydrogen atom in has one bonding pair. The formal charge on each hydrogen atom is therefore
formal charge(H)=1−(0+2/2)=0
The formal charges on the atoms in the NH4+ ion are thus
Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. In this case, the sum of the formal charges is 0 + 1 + 0 + 0 + 0 = +1.
Example
Write the formal charges on all atoms in BH4−.
Show AnswerIf an atom in a molecule or ion has the number of bonds that is typical for that atom (e.g., four bonds for carbon), its formal charge is zero.
Using Formal Charges to Distinguish between Lewis Structures
As an example of how formal charges can be used to determine the most stable Lewis structure for a substance, we can compare two possible structures for CO2. Both structures conform to the rules for Lewis electron structures.
1. C is less electronegative than O, so it is the central atom.
2. C has 4 valence electrons and each O has 6 valence electrons, for a total of 16 valence electrons.
3. Placing one electron pair between the C and each O gives O–C–O, with 12 electrons left over.
4. Dividing the remaining electrons between the O atoms gives three lone pairs on each atom:
This structure has an octet of electrons around each O atom but only 4 electrons around the C atom.
5. No electrons are left for the central atom.
6. To give the carbon atom an octet of electrons, we can convert two of the lone pairs on the oxygen atoms to bonding electron pairs. There are, however, two ways to do this. We can either take one electron pair from each oxygen to form a symmetrical structure or take both electron pairs from a single oxygen atom to give an asymmetrical structure:
Both Lewis electron structures give all three atoms an octet. How do we decide between these two possibilities? The formal charges for the two Lewis electron structures of CO2 are as follows:
Both Lewis structures have a net formal charge of zero, but the structure on the right has a +1 charge on the more electronegative atom (O). Thus the symmetrical Lewis structure on the left is predicted to be more stable, and it is, in fact, the structure observed experimentally. Remember, though, that formal charges do not represent the actual charges on atoms in a molecule or ion. They are used simply as a bookkeeping method for predicting the most stable Lewis structure for a compound.
Note:
The Lewis structure with the set of formal charges closest to zero is usually the most stable.
Examples
The thiocyanate ion (SCN−), which is used in printing and as a corrosion inhibitor against acidic gases, has at least two possible Lewis electron structures. Draw two possible structures, assign formal charges on all atoms in both, and decide which is the preferred arrangement of electrons.
Given: chemical species
Asked for: Lewis electron structures, formal charges, and preferred arrangement
Strategy:
A Use the step-by-step procedure to write two plausible Lewis electron structures for SCN−.
B Calculate the formal charge on each atom using formal charge = valence e−−(free atom)(non−bonding e− + bonding e−/2)
C Predict which structure is preferred based on the formal charge on each atom and its electronegativity relative to the other atoms present.
Show AnswerA Possible Lewis structures for the SCN− ion are as follows:
B We must calculate the formal charges on each atom to identify the more stable structure. If we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of bonds typical for carbon, so it has a formal charge of zero. Continuing with sulfur, we observe that in (a) the sulfur atom shares one bonding pair and has three lone pairs and has a total of six valence electrons. The formal charge on the sulfur atom is therefore 6−(6+2/2)=−1 and 5−(4+4/2)=−1. In (c), nitrogen has a formal charge of −2.
C Which structure is preferred? Structure (b) is preferred because the negative charge is on the more electronegative atom (N), and it has lower formal charges on each atom as compared to structure (c): 0, −1 versus +1, −2.
Example
Salts containing the fulminate ion (CNO−) are used in explosive detonators. Draw three Lewis electron structures for CNO− and use formal charges to predict which is more stable. (Note: N is the central atom.)
Show AnswerThe second structure is predicted to be more stable.
Contributors
- Anonymous
| Writing Lewis Structures by Trial and Error | A Step-By-Step Approach to Writing Lewis Structures | Drawing Skeleton Structures |
| Molecules that Contain Too Many or Not Enough Electrons | Resonance Hybrids | Formal Charge |
Writing Lewis Structures by Trial and Error
The Lewis structure of a compound can be generated by trial and error. We start by writing symbols that contain the correct number of valence electrons for the atoms in the molecule. We then combine electrons to form covalent bonds until we come up with a Lewis structure in which all of the elements (with the exception of the hydrogen atoms) have an octet of valence electrons.
Example: Let's apply the trial and error approach to generating the Lewis structure of carbon dioxide, CO2. We start by determining the number of valence electrons on each atom from the electron configurations of the elements. Carbon has four valence electrons, and oxygen has six.
C: [He] 2s2 2p2
O: [He] 2s2 2p4
We can symbolize this information as shown at the top of the figure below. We now combine one electron from each atom to form covalent bonds between the atoms. When this is done, each oxygen atom has a total of seven valence electrons and the carbon atom has a total of six valence electrons. Because none of these atoms have an octet of valence electrons, we combine another electron on each atom to form two more bonds. The result is a Lewis structure in which each atom has an octet of valence electrons.
The trial-and-error method for writing Lewis structures can be time consuming. For all but the simplest molecules, the following step-by-step process is faster.
Step 1: Determine the total number of valence electrons.
Step 2: Write the skeleton structure of the molecule.
Step 3: Use two valence electrons to form each bond in the skeleton structure.
Step 4: Try to satisfy the octets of the atoms by distributing the remaining valence electrons as nonbonding electrons.
The first step in this process involves calculating the number of valence electrons in the molecule or ion. For a neutral molecule this is nothing more than the sum of the valence electrons on each atom. If the molecule carries an electric charge, we add one electron for each negative charge or subtract an electron for each positive charge.
Example: Let's determine the number of valence electrons inthe chlorate (ClO3-) ion.
A chlorine atom (Group VIIA) has seven valence electrons and each oxygen atom (Group VIA) has six valence electrons. Because the chlorate ion has a charge of -1, this ion contains one more electron than a neutral ClO3 molecule. Thus, the ClO3- ion has a total of 26 valence electrons.
ClO3-: 7 + 3(6) + 1 = 26
The second step in this process involves deciding which atoms in the molecule are connected by covalent bonds. The formula of the compound often provides a hint as to the skeleton structure. The formula for the chlorate ion, for example, suggests the following skeleton structure.
The third step assumes that the skeleton structure of the molecule is held together by covalent bonds. The valence electrons are therefore divided into two categories: bonding electrons and nonbonding electrons. Because it takes two electrons to form a covalent bond, we can calculate the number of nonbonding electrons in the molecule by subtracting two electrons from the total number of valence electrons for each bond in the skeleton structure.
There are three covalent bonds in the most reasonable skeleton structure for the chlorate ion. As a result, six of the 26 valence electrons must be used as bonding electrons. This leaves 20 nonbonding electrons in the valence shell.
| 26 valence electrons |
| - 6 bonding electrons |
| 20 nonbonding electrons |
The nonbonding valence electrons are now used to satisfy the octets of the atoms in the molecule. Each oxygen atom in the ClO3- ion already has two electrons the electrons in the Cl-O covalent bond. Because each oxygen atom needs six nonbonding electrons to satisfy its octet, it takes 18 nonbonding electrons to satisfy the three oxygen atoms. This leaves one pair of nonbonding electrons, which can be used to fill the octet of the central atom.
The most difficult part of the four-step process in the previous section is writing the skeleton structure of the molecule. As a general rule, the less electronegative element is at the center of the molecule.
Example: The formulas of thionyl chloride (SOCl2) and sulfuryl chloride (SO2Cl2) can be translated into the following skeleton structures.
It is also useful to recognize that the formulas for complex molecules are often written in a way that hints at the skeleton structure of the molecule.
Example: Dimethyl ether is often written as CH3OCH3, which translates into the following skeleton structure.
Finally, it is useful to recognize that many compounds that are acids contain O-H bonds.
Example: The formula of acetic acid is often written as CH3CO2H, because this molecule contains the following skeleton structure.
Too Few Electrons
Occasionally we encounter a molecule that doesn't seem to have enough valence electrons. If we can't get a satisfactory Lewis structure by sharing a single pair of electrons, it may be possible to achieve this goal by sharing two or even three pairs of electrons.
Example: Consider formaldehyde (H2CO) which contains 12 valence electrons.
H2CO: 2(1) + 4 + 6 = 12
The formula of this molecule suggests the following skeleton structure.
There are three covalent bonds in this skeleton structure, which means that six valence electrons must be used as bonding electrons. This leaves six nonbonding electrons. It is impossible, however, to satisfy the octets of the atoms in this molecule with only six nonbonding electrons. When the nonbonding electrons are used to satisfy the octet of the oxygen atom, the carbon atom has a total of only six valence electrons.
We therefore assume that the carbon and oxygen atoms share two pairs of electrons. There are now four bonds in the skeleton structure, which leaves only four nonbonding electrons. This is enough, however, to satisfy the octets of the carbon and oxygen atoms.
Every once in a while, we encounter a molecule for which it is impossible to write a satisfactory Lewis structure.
Example: Consider boron trifluoride (BF3) which contains 24 valence electrons.
BF3: 3 + 3(7) = 24
There are three covalent bonds in the most reasonable skeleton structure for the molecule. Because it takes six electrons to form the skeleton structure, there are 18 nonbonding valence electrons. Each fluorine atom needs six nonbonding electrons to satisfy its octet. Thus, all of the nonbonding electrons are consumed by the three fluorine atoms. As a result, we run out of electrons while the boron atom has only six valence electrons.
The elements that form strong double or triple bonds are C, N, O, P, and S. Because neither boron nor fluorine falls in this category, we have to stop with what appears to be an unsatisfactory Lewis structure.
Too Many Electrons
It is also possible to encounter a molecule that seems to have too many valence electrons. When that happens, we expand the valence shell of the central atom.
Example: Consider the Lewis structure for sulfur tetrafluoride (SF4) which contains 34 valence electrons.
Lewis Dot Diagram Solver
SF4: 6 + 4(7) = 34
There are four covalent bonds in the skeleton structure for SF4. Because this requires using eight valence electrons to form the covalent bonds that hold the molecule together, there are 26 nonbonding valence electrons.
Each fluorine atom needs six nonbonding electrons to satisfy its octet. Because there are four of these atoms, so we need 24 nonbonding electrons for this purpose. But there are 26 nonbonding electrons in this molecule. We have already satisfied the octets for all five atoms, and we still have one more pair of valence electrons. We therefore expand the valence shell of the sulfur atom to hold more than eight electrons.
This raises an interesting question: How does the sulfur atom in SF4 hold 10 electrons in its valence shell? The electron configuration for a neutral sulfur atom seems to suggest that it takes eight electrons to fill the 3s and 3p orbitals in the valence shell of this atom. But let's look, once again, at the selection rules for atomic orbitals. According to these rules, the n = 3 shell of orbitals contains 3s, 3p, and 3d orbitals. Because the 3d orbitals on a neutral sulfur atom are all empty, one of these orbitals can be used to hold the extra pair of electrons on the sulfur atom in SF4.
S: [Ne] 3s2 3p4 3d0
| Practice Problem 3: Write the Lewis structure for xenon tetrafluoride (XeF4). |
Lewis Dot Structure Calc
Two Lewis structures can be written for sulfur dioxide.
The only difference between these Lewis structures is the identity of the oxygen atom to which the double bond is formed. As a result, they must be equally satisfactory representations of the molecule.
Lewis Electron Dot Structure Calculator Equation
Interestingly enough, neither of these structures is correct. The two Lewis structures suggest that one of the sulfur-oxygen bonds is stronger than the other. There is no difference between the length of the two bonds in SO2, however, which suggests that the two sulfur-oxygen bonds are equally strong.
When we can write more than one satisfactory Lewis structure, the molecule is an average, or resonance hybrid, of these structures. The meaning of the term resonance can be best understood by an analogy. In music, the notes in a chord are often said to resonate they mix to give something that is more than the sum of its parts. In a similar sense, the two Lewis structures for the SO2 molecule are in resonance. They mix to give a hybrid that is more than the sum of its components. The fact that SO2 is a resonance hybrid of two Lewis structures is indicated by writing a double-headed arrow between these Lewis structures, as shown in the figure above.
| Practice Problem 4: Write the Lewis structures for the acetate ion, CH3CO2-. |
Formal Charge
It is sometimes useful to calculate the formal charge on each atom in a Lewis structure. The first step in this calculation involves dividing the electrons in each covalent bond between the atoms that form the bond. The number of valence electrons formally assigned to each atom is then compared with the number of valence electrons on a neutral atom of the element. If the atom has more valence electrons than a neutral atom, it is assumed to carry a formal negative charge. If it has fewer valence electrons it is assigned a formal positive charge.
| Practice Problem 5: The formula of the amino acid known as glycine is often written as H3N+CH2CO2-. Use the concept of formal charge to explain the meaning of the positive and negative signs in the following Lewis structure. |